Abstract
Introduction- Several in vivo studies and clinical studies have demonstrated extracellular DNA as a mediator of coagulation and this effect was reversed by the administration of DNA degrading enzyme DNase I. However, there is no clear understanding of the mechanism by which extracellular DNA activates coagulation in vitro. Conventionally, it was thought to be the activator of the contact pathway. But recent studies have shown that extracellular DNA isolated without contaminants like silica particles is a weak activator of the contact pathway. In this study, we have investigated the mechanism by which extracellular DNA is contributing to coagulation. Corroborating with recent results, we show that extracellular genomic DNA is a weak activator contact pathway. We determined that extracellular DNA accelerate fibrinogen polymerization by thrombin via a possible template mechanism. Our biophysical studies corroborate the interaction of DNA, thrombin, and fibrinogen. Understanding the mechanism of DNA induced blood coagulation will help address the gaps in the literature as well as develop inhibitors against DNA- mediated thrombosis.
Methods- Silica-free extracellular DNA was purified with the PAXgene™ Blood DNA Kit. Contact activation in plasma was measured by monitoring the cleavage of the substrate S2302. To study the contact independent activation of plasma clotting by extracellular DNA, 1.5 µM Corn Trypsin Inhibitor (CTI) was applied to the plasma. Next, acceleration of fibrinogen polymerization by thrombin in presence of extracellular DNA was measured by monitoring the absorbance of 350 nm. Interaction of DNA with fibrinogen and thrombin in phosphate buffer was determined by CD spectroscopy.
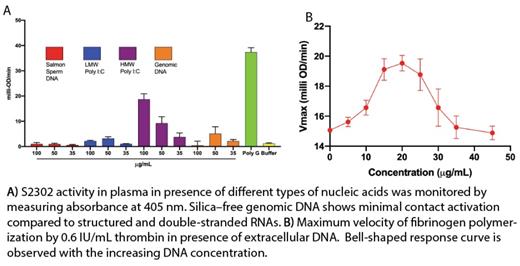
Results- Our results show that silica-free extracellular genomic DNA is a weak activator of the contact pathway of coagulation [Fig-A]. Moreover, genomic DNA accelerated the plasma clotting even when the contact pathway was inhibited with CTI indicating a contact independent mechanism of the procoagulant activity of extracellular DNA. Interestingly, the presence of extracellular DNA accelerated the polymerization of fibrinogen in presence of thrombin [Fig-B]. A bell-shaped dose-response curve for extracellular DNA indicates a likely template mechanism in which both thrombin and fibrinogen could assemble on the DNA molecule. These results are supported by the results from the CD spectroscopy studies where an alteration of the structure of fibrinogen and thrombin can be noticed in presence of extracellular DNA. Confocal studies further corroborate this observation. Our results also show different nucleic acids activate coagulation via different pathways.
Significance- Procoagulant activity of extracellular DNA is demonstrated in several mouse models. However, a clear understanding of the mechanism of procoagulant activity of DNA in vitro has been challenging due to the caveats in the isolation of extracellular DNA where it is often contaminated with silica particles. Here we show a novel procoagulant mechanism of cell- free DNA where it augments the polymerization of fibrinogen by thrombin. These results provide insights into the mechanism of procoagulant activity of DNA which is key to develop therapeutics against procoagulant DNA.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal